Gene therapy for hemophilia is revolutionizing treatment options for those affected by this genetic bleeding disorder. Historically, hemophilia treatment has relied on regular injections of clotting factor, which many patients endure for a lifetime. However, recent advancements in gene therapy innovations such as Hemgenix offer a new ray of hope by potentially providing a long-lasting solution. This therapy, particularly beneficial for hemophilia B patients, aims to correct the underlying genetic defects in a single treatment. Despite the promise it holds, discussions surrounding gene therapy cost raise questions about accessibility and affordability for patients eager for a reprieve from their lifelong struggles.
The emerging field of genetic interventions is paving the way for groundbreaking alternatives in managing hemophilia. With the advent of innovative therapies like Hemgenix, patients now have a chance to address the root genetic issues causing hemophilia, particularly in those suffering from hemophilia B. These therapies challenge traditional methods of hemophilia treatment by offering a potentially one-time solution that could drastically reduce the need for ongoing treatments. However, as these advanced options surface, the financial implications, particularly the gene therapy cost, become increasingly critical in determining their widespread adoption and implementation. Such advancements not only inspire hope but also highlight the complex intersection of science, ethics, and healthcare economics.
Understanding Hemophilia: A Lifelong Challenge
Hemophilia is a genetic disorder affecting the body’s ability to produce blood clots, leading to excessive bleeding even from minor injuries. Patients like Terence Blue manage their condition from a young age, navigating hospital visits and daily treatments to infuse clotting factors. Despite advancements in medical technology, living with hemophilia means constant vigilance and lifestyle adjustments. Regular injections of clotting factor become a part of life, making every patient’s journey unique as they strive for normalcy amidst the limitations imposed by their condition.
The emotional and psychological toll of hemophilia is profound. Individuals often deal with the social implications of their condition, having to explain their limitations to friends and family, which can inadvertently lead to isolation. Each bleed brings anxiety and uncertainty, requiring not just medical management but also mental resilience. This relentless nature of hemophilia underlines the necessity for advanced treatment options that can provide lasting solutions, such as gene therapy.
Gene Therapy for Hemophilia: A Breakthrough in Treatment
Gene therapy has emerged as a transformative approach for treating hemophilia, particularly with innovations like Hemgenix for hemophilia B. This cutting-edge therapy works by introducing corrected copies of the gene responsible for producing clotting factor IX, which is deficient in patients. The ability to provide a potential one-time treatment that alleviates the daily burden of injections is revolutionary and offers hope for improved quality of life, minimizing the risk of spontaneous bleeding.
Patients who have undergone gene therapy often report a significant reduction in the need for clotting factor infusions, with clinical trials showing remarkable success rates. The emotional relief of not having to worry constantly about bleeds can dramatically improve a patient’s psychological well-being. As Terence Blue’s experience illustrates, the journey of receiving gene therapy not only aims for a biological correction but also rejuvenates a sense of freedom many patients have longed for.
Hemgenix: The Latest in Gene Therapy Innovations
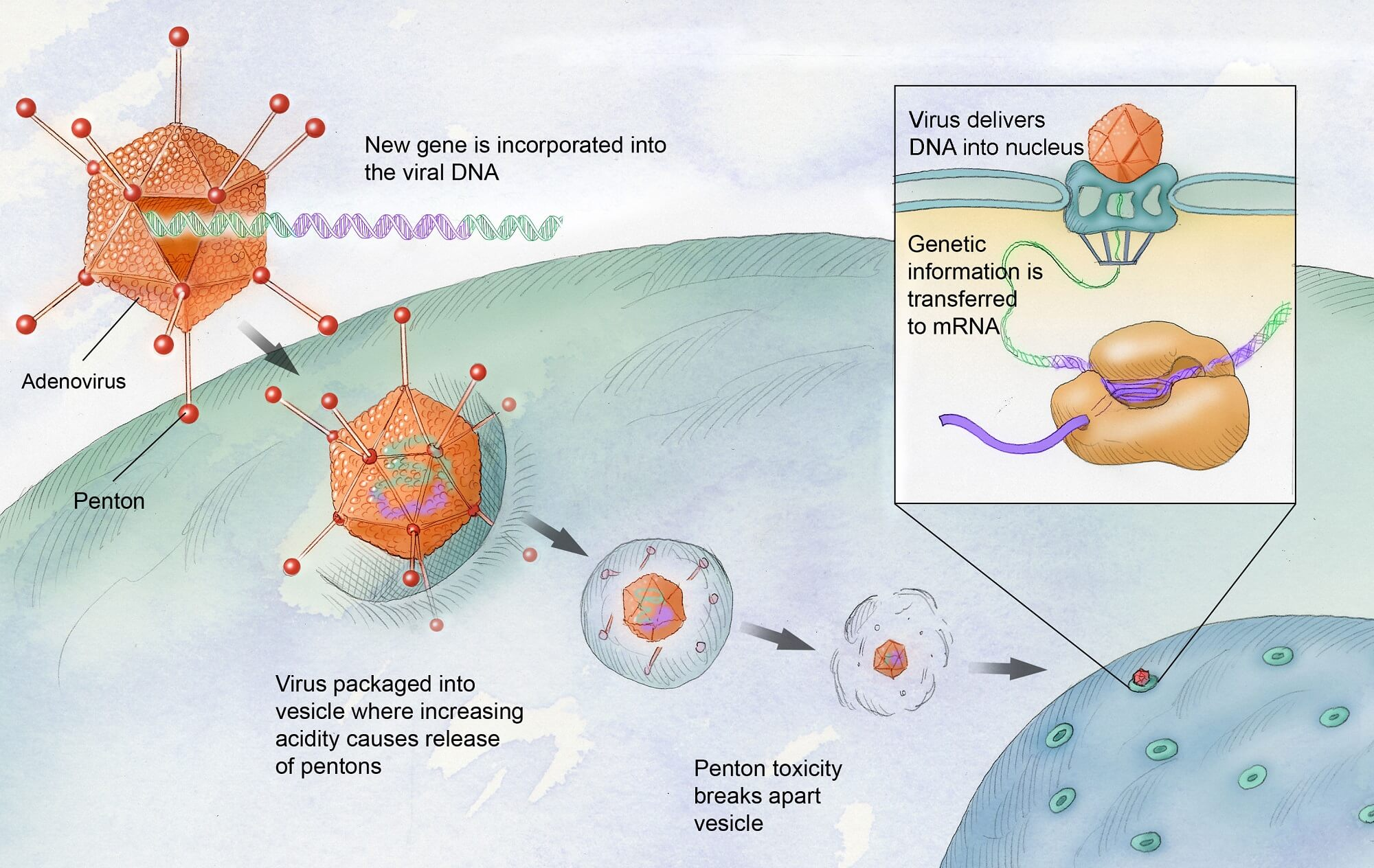
Hemgenix represents a major milestone in the field of gene therapy, specifically designed for patients suffering from hemophilia B. Approved by the FDA in November 2022, this therapy utilizes an adeno-associated virus to deliver genetic instruction directly to liver cells, where the body naturally synthesizes clotting factors. The challenges of designing effective vectors that can safely deliver genetic payloads have been met with this innovative approach, promising not just safety but significant efficacy.
The excitement surrounding Hemgenix is palpable among the hemophilia community. With the prospect of long-term benefits from a single infusion, it shifts the paradigm of hemophilia treatment from routine Factor IX injections to a more sustainable solution. The hope is that therapies like Hemgenix will pave the way for future advancements, inspiring additional research and funding that can lead to broader applications of gene therapy in treating various genetic disorders.
Market Challenges of Gene Therapy: Balancing Innovation and Cost
While gene therapies like Hemgenix offer groundbreaking treatments, they come with formidable market challenges. The high price point of gene therapy — approximately $3.5 million per treatment — poses significant concerns for patients and healthcare systems alike. Insurance providers must navigate the complexities of covering single-dose treatments versus chronic therapies, which complicates patient access. Furthermore, companies face pressure to justify the costs in a market that requires return on research investment.
Reducing the prices of gene therapies while ensuring they remain profitable is a tough balancing act. The financial realities mean that not every innovative treatment can become widely adopted, leading some promising therapies to exit the market prematurely. As health systems cope with the economic implications of these therapies, there is a crucial need for transparent discussions about value, efficacy, and patient benefits, which can ultimately shape the healthcare landscape for hemophilia treatment.
Patient Perspectives on Gene Therapy: A New Era of Hope
The patient experience is the core of advancements like gene therapy for hemophilia. Individuals like Terence Blue express feelings of hope and relief at the prospect of reducing their treatment burden. The morning of his therapy, anticipation mixed with anxiety became a pivotal moment in his life, representing not just medical treatment but a shift in lifestyle. This underscores the importance of patient education and emotional support as they embark on such transformative therapies.
Patients often navigate complex decisions regarding whether to pursue new treatments. Conversations with medical professionals about the risks and benefits of gene therapy are crucial. With a treatment like Hemgenix which potentially offers long-term results, patients are empowered to consider options that can alter their lives significantly. The anticipation of a possible cure aligns with the inherent desire of every patient to lead a normal, unencumbered life.
Future Directions in Hemophilia Treatment
Looking to the future, the landscape of hemophilia treatment is rapidly evolving. Advances in gene therapy are poised to lead to new innovations that can further enhance patient outcomes. As ongoing research unravels the complexities of genetic diseases, therapies aimed at other forms of hemophilia and related disorders are on the horizon, aiming for broader applications beyond current treatments.
Moreover, collaboration between researchers, healthcare providers, and patients will be key in shaping the future of hemsophilia therapies. Continuous investment in scientific research will foster an environment where innovative treatments can flourish, providing hope and potentially life-altering solutions for those living with hemophilia.
The Role of Clinical Trials in Advancing Gene Therapy
Clinical trials are vital for validating the efficacy and safety of new treatments such as gene therapy. Rigorous trials provide the necessary data to ensure treatments like Hemgenix meet established medical standards before reaching the wider patient population. The participatory aspect of clinical trials also engages patients directly in the research process, allowing them to contribute actively to the development of therapies that could change their lives.
As gene therapy research progresses, ongoing clinical trials will continue to be instrumental in discovering new methods for delivering effective treatments. Transparency in results and continued discussion about trial outcomes will foster trust between medical researchers and the community, bolstering support for emerging therapies as they become available.
Regulatory Landscape for Gene Therapy Approvals
The regulatory landscape plays a critical role in determining the availability of innovative therapies like gene therapy for hemophilia. Agencies such as the FDA have stringent guidelines in place to ensure that treatments are safe and effective before approval for public use. However, the urgency for new solutions often leads to calls for expedited approval processes, particularly for life-changing treatments that could mitigate the burdens of chronic conditions.
Navigating the regulatory framework while accommodating the need for timely access to cutting-edge therapies is a challenge faced by both researchers and policy makers. Striking a balance between thorough review and expedited approval processes remains essential to ensure that patients have access to effective treatments without compromising safety and efficacy.
Living with Hemophilia: The Transition to a New Normal
For many hemophilia patients, the prospect of gene therapy brings the hope of transitioning to a new normal, free from the constant need for factor infusions. As individuals begin to experience the effects of therapies like Hemgenix, they are not just undergoing physical changes but are also reshaping their day-to-day lives. The idea of living without daily shots and the anxiety of potential bleeds allows patients to envision a brighter future.
Adapting to this new normal encompasses more than just medical change; it involves re-establishing social connections and reintegrating into everyday life activities without the burden of managing a chronic condition. The emotional component of this transition is significant, highlighting the necessity for support systems that foster encouragement and guidance as patients navigate this newfound freedom.
Frequently Asked Questions
What is gene therapy for hemophilia and how does it work?
Gene therapy for hemophilia is a revolutionary treatment that aims to correct the genetic mutations causing the disorder. This therapy involves introducing a healthy copy of the gene responsible for producing clotting factors directly into the patient’s liver using a modified virus. Hemgenix, a specific gene therapy for hemophilia B, has shown significant promise by increasing the production of clotting factor IX, reducing or eliminating the need for regular injections.
How does Hemgenix improve hemophilia treatment outcomes?
Hemgenix represents a major advancement in hemophilia treatment by providing a one-time infusion that can potentially lead to long-term production of clotting factor IX. In clinical trials, about 94% of patients treated with Hemgenix did not require factor IX prophylaxis three years later, demonstrating its effectiveness in significantly improving quality of life for hemophilia B patients.
What are the potential side effects of gene therapy for hemophilia B?
While gene therapy for hemophilia B, like Hemgenix, is a promising treatment, it can have side effects. Common side effects observed include elevated liver enzymes, necessitating monitoring and possible steroid treatment. Patients may experience mild allergic reactions, but serious adverse effects are generally rare. Close supervision by a healthcare team is crucial during the treatment process.
What is the cost of gene therapy for hemophilia, specifically Hemgenix?
The cost of gene therapy for hemophilia, such as Hemgenix, is quite high, with a list price around $3.5 million. However, insurance companies often negotiate lower rates. The high price reflects the complex development process of gene therapies, which aim to provide long-term benefits through potentially curative approaches, rather than ongoing treatment costs like traditional hemophilia therapies.
Can gene therapy be considered a cure for hemophilia?
Although gene therapy for hemophilia shows incredible promise and can dramatically reduce the need for factor IX injections, it is not classified as a definitive cure. Treatments like Hemgenix can result in lasting improvements that may exceed years, but ongoing monitoring and research are necessary to understand the full extent of the benefits and any potential long-term effects.
| Key Point | Details |
|---|---|
| Introduction of Gene Therapy | Terence Blue was the first patient in New England to receive Hemgenix, a gene therapy for hemophilia B. |
| Background of Hemophilia | Hemophilia is a blood disorder requiring regular clotting factor injections; about 33,000 men in the U.S. live with it. |
| Benefits of Gene Therapy | Gene therapy aims to provide long-lasting benefits and potentially eliminate the need for constant injection. |
| Challenges and Market Dynamics | High treatment costs can hinder market growth and patient access; for instance, Hemgenix costs $3.5 million. |
| Treatment Administration | The therapy uses modified viruses to deliver corrected genes to the liver, stimulating factor IX production. |
| Patient Experience | Blue’s factor IX level improved significantly post-treatment, demonstrating the potential efficacy of gene therapy. |
Summary
Gene therapy for hemophilia represents a revolutionary advancement in the treatment of this genetic blood disorder. Through innovative approaches like Hemgenix, patients can potentially experience improved quality of life without the hassle of daily injections. Terence Blue’s experience illustrates the hope and tangible benefits this therapy can bring, reducing dependence on traditional treatment methods. Continued research and development in this field hold promise not only for hemophilia patients but also for many other genetic disorders, paving the way for a new era in medicine where gene therapies could become standard care.