Alzheimer’s disease research is rapidly evolving, driven by groundbreaking studies that explore the intricate workings of the brain’s immune system, particularly the role of microglial cells. These cells are crucial for maintaining brain health as they patrol for damage and clear out dead cells. However, irregularities in their functioning can contribute to Alzheimer’s and other neurodegenerative diseases, making this research vital for developing new Alzheimer’s treatments. Under the leadership of neuroscientist Beth Stevens, significant advancements have been made, establishing potential new biomarkers for early detection. As the incidence of Alzheimer’s continues to rise among aging populations, the implications of this research could change the course of treatment for millions.
Innovations in Alzheimer’s disease research encompass a vital exploration of the brain’s immune cells and their influence on cognitive decline. Known as microglia, these cells are central to understanding how the brain responds to injury and disease. Researchers like Beth Stevens are at the forefront of this work, revealing how errors in microglial function may lead to debilitating conditions, including Alzheimer’s. By identifying these malfunctioning pathways, scientists aim to unlock new therapies to combat neurodegenerative disorders. As the landscape of treatment options expands, the urgency to address the needs of those affected by Alzheimer’s only grows stronger.
The Role of Microglial Cells in Alzheimer’s Disease
Microglial cells are vital components of the brain’s immune system, responsible for maintaining neurological health by removing cellular debris and regulating synaptic connections. In the context of Alzheimer’s disease, these cells play a critical role in brain function and pathology. Research has shown that microglia can become activated in response to neurodegenerative processes, which can lead to both protective and harmful effects. Aberrant microglial function, particularly in synaptic pruning, has been identified as a key factor contributing to Alzheimer’s and other neurodegenerative diseases, highlighting their dual nature in both supporting and hindering neuronal health.
Recent studies, particularly those led by Beth Stevens, underscore the importance of understanding microglial behavior and its implications for Alzheimer’s treatment. By elucidating how these immune cells interact with synapses, researchers aim to develop targeted therapies to restore normal pruning processes that become dysfunctional in conditions like Alzheimer’s. Innovations stemming from these findings could lead to breakthroughs in therapeutic strategies, with potential applications in early diagnosis and intervention for neurodegenerative diseases.
Alzheimer’s disease research has increasingly focused on how microglial dysfunction may exacerbate cognitive decline, providing insight into preventive measures and treatment options. As our understanding of microglial roles deepens, the prospect of leveraging their pathways for Alzheimer’s treatment grows more promising. Researchers, including those at the Stevens Lab, are uncovering the complexities of microglial involvement in synaptic health—pointing to the necessity of integrating immune response studies into broader Alzheimer’s research.
Ultimately, the ongoing exploration of microglial cell functions leads not only to a better grasp of Alzheimer’s mechanisms but also fosters hope for more effective interventions. As scientists continue to decipher the intricate relationships between neuroinflammation, synaptic pruning, and Alzheimer’s pathophysiology, the potential for novel therapeutic strategies becomes increasingly tangible.
Innovations in Alzheimer’s Treatment Through Basic Science
The foundational research carried out in labs like Stevens’ at Boston Children’s Hospital exemplifies how basic science paves the way for innovative treatments for Alzheimer’s disease. By investigating the underlying biology of microglial cells and neurodegenerative mechanisms, Stevens and her team are redefining potential approaches to alleviating the impacts of Alzheimer’s. Their findings not only advance scientific knowledge but also facilitate the development of therapeutic avenues that might one day result in effective Alzheimer’s treatments.
Funding from federal agencies has been crucial in driving such exploratory research, as mentioned by Stevens. This financial support allows scientists to engage in curiosity-driven projects that may initially appear distant from direct clinical applications. However, the unexpected outcomes from these studies often lead to breakthroughs that hold significant promise for disease treatment and management. The case of microglial research is a prime example of how sustained basic science efforts can ultimately improve therapeutic options for millions affected by Alzheimer’s.
Moreover, as discussed in Stevens’ perspectives, the synergy between basic research and clinical translation is essential for addressing neurodegenerative diseases like Alzheimer’s. By investing in understanding the fundamental processes within the brain’s immune system, scientists glean insights that could inform targeted therapeutic strategies. The interplay of innovation, meticulous scientific inquiry, and federal support is likely to catalyze more effective interventions for Alzheimer’s disease in the foreseeable future.
As the number of Alzheimer’s cases continues to rise, driven by an aging population, the urgency for new treatment modalities is becoming increasingly critical. The groundwork laid by researchers like Beth Stevens not only contributes to the immediate field of neuroimmunology but also inspires future generations of scientists to tackle the complexities associated with Alzheimer’s disease and other neurodegenerative disorders.
Exploring New Biomarkers for Early Detection of Alzheimer’s
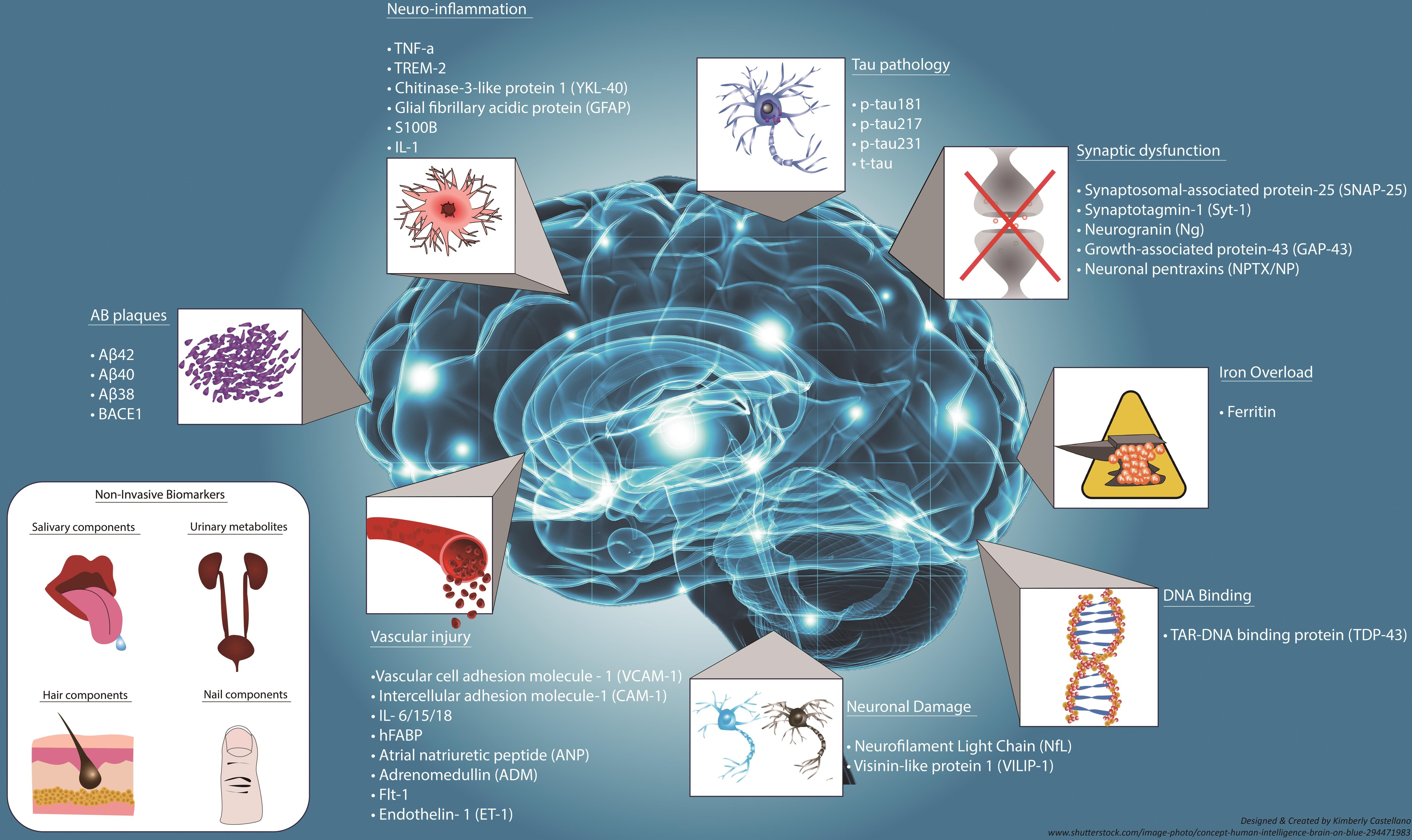
The quest for effective biomarkers is crucial in the fight against Alzheimer’s disease; early detection can significantly sway treatment outcomes and improve the quality of life for many patients. New research emerging from institutions such as the Broad Institute, in collaboration with experts like Beth Stevens, seeks to establish biomarkers that accurately signal the onset of Alzheimer’s. Identifying specific microglial activity as a potential biomarker for Alzheimer’s presents an exciting avenue for early intervention, allowing for timely therapeutic measures to be taken as the disease progresses.
Establishing reliable biomarkers is not just advantageous for early detection but also critical for tracking disease progression, assessing treatment efficacy, and developing individualized therapeutic strategies. By understanding how microglia interact with Alzheimer’s pathology, researchers hope to pinpoint unique signatures within the brain that indicate underlying neurodegenerative activity. Through these efforts, scientists are one step closer to devising comprehensive frameworks for Alzheimer’s diagnosis, paving the way for interventions that can alter disease trajectories.
Additionally, the development of biomarkers leverages the tools of modern science, including genetic and molecular techniques, to provide deeper insights into Alzheimer’s pathology. As research advances, it becomes increasingly evident that a multidisciplinary approach is necessary, merging neurobiology with diagnostics to formulate a standardized method for clinical application. This would greatly benefit not only existing patients but also at-risk populations by promoting preventive measures against Alzheimer’s.
As researchers continue their work on microglial function and biomarkers, the goal remains to create a healthcare landscape where Alzheimer’s disease can be detected early and managed effectively. With the emergence of advanced technologies and methodologies, the potential for revolutionizing Alzheimer’s detection and treatment appears promising, underscoring the vital role of ongoing research in improving patient outcomes.
The Impact of Aging on Neurodegenerative Diseases
Aging is the most significant risk factor for developing neurodegenerative diseases, particularly Alzheimer’s. As the global population ages, the incidence of Alzheimer’s disease is anticipated to climb substantially. Understanding the biological mechanisms linking aging to neurodegenerative processes, particularly in relation to microglial functionality, is imperative. Studies indicate that the aging brain undergoes various changes that can alter microglial activation patterns, potentially leading to an amplified inflammatory response that contributes to neurodegeneration.
Research conducted by scientists like Beth Stevens delves into how age-related changes in microglial behavior can influence the development and progression of Alzheimer’s. The interactions between age, microglial activity, and synaptic health are complex, requiring thorough investigation to unravel. By targeting these age-related mechanisms, researchers aim to identify therapeutic strategies that could mitigate the heightened risk of Alzheimer’s among the elderly, thus improving care and outcomes for this vulnerable population.
Additionally, these age-centric studies have broader implications for the field of neurodegenerative disease research. By focusing on aging’s impact, scientists can better understand the thresholds at which neurodegenerative diseases manifest and progress. This knowledge is essential for developing preventive strategies that could protect at-risk individuals before Alzheimer’s manifests clinically.
In summary, an exploration of aging in the context of Alzheimer’s and other neurodegenerative diseases highlights the importance of adaptive research strategies that consider the unique biological landscape of older adults. It emphasizes the need for continued interdisciplinary collaboration in pursuit of innovative solutions to one of the most pressing health challenges of our time.
Neuroscience: A Pathway to Understanding Neurodegenerative Diseases
Neuroscience plays an instrumental role in illuminating the complexities of neurodegenerative diseases, particularly Alzheimer’s. By harnessing cutting-edge technologies and methodologies, scientists are gaining new perspectives on how diseases such as Alzheimer’s and Huntington’s affect neuronal health. Research initiatives, like those spearheaded by Beth Stevens, exemplify how a dedication to basic neuroscience can lead to profound implications for understanding disease mechanisms and therapeutic developments.
One fundamental aspect of neuroscience research involves studying the brain’s immune system, particularly the pivotal role of microglial cells in maintaining homeostasis and responding to injury. Neurodegenerative diseases often disrupt this balance, leading to accelerated neuronal deterioration. Understanding these dynamics allows researchers to explore innovative avenues for treatment that target the root causes of disease rather than merely addressing symptoms.
Moreover, interdisciplinary collaboration between neuroscientists, immunologists, and clinicians can catalyze significant advancements in understanding and treating neurodegenerative diseases. By pooling expertise and resources, these collaborations foster an enriched environment for discovering new therapeutics and insights into Alzheimer’s disease. The focus on basic science serves to inform future clinical applications, creating a continuum of research-to-practice that is essential for meaningful progress.
Ultimately, the commitment to neuroscience as the foundation for tackling neurodegenerative diseases signifies a critical approach in combating Alzheimer’s. The integration of comprehensive research methodologies, including behavioral studies and molecular studies on microglial function, represents the future of disease understanding and treatment.
The Significance of Funding in Alzheimer’s Research
In the landscape of Alzheimer’s research, funding plays a pivotal role in driving significant breakthroughs. As highlighted by Beth Stevens, the support from federal agencies like the National Institutes of Health has been instrumental in advancing our understanding of neurodegenerative diseases. This funding enables researchers to embark on ambitious projects that delve into the intricacies of Alzheimer’s disease, exploring areas such as microglial behavior and the fundamental biology of neuronal health.
Sustained financial investments in research not only nurture the development of innovative therapeutic strategies but also bolster collaborative efforts across disciplines. The interdependence between federal funding and scientific discovery cannot be overstated—without adequate resources, many promising research projects would remain unfunded, resulting in lost opportunities for discovery that could transform Alzheimer’s treatment paradigms.
Moreover, understanding the allocation of funding can shed light on the priorities within the field of Alzheimer’s research. As the need for effective treatments grows, emphasizing support for projects investigating the role of microglial cells in neurodegenerative diseases will likely yield beneficial insights, unlocking new avenues for intervention. By prioritizing research that tackles pressing concerns in Alzheimer’s, funding agencies can catalyze progress and inspire future scientific inquiry.
In conclusion, the significance of funding in Alzheimer’s research cannot be understated. It serves not only as a catalyst for scientific progress but also as a barometer for the direction that research takes in addressing critical challenges associated with neurodegenerative diseases. A sustained commitment to funding is essential for the ongoing pursuit of innovative solutions to combat Alzheimer’s and improve outcomes for those affected.
The Future of Alzheimer’s Disease Research and Treatment
As we look towards the future of Alzheimer’s disease research, the prospects for innovative treatment options and early intervention strategies are increasingly optimistic. Driven by advancements in scientific understanding, particularly regarding microglial function, researchers are piecing together the framework for comprehensive approaches to manage this complex disease. The work of pioneers like Beth Stevens highlights the importance of integrating basic research with clinical applications, paving pathways for breakthroughs that could redefine Alzheimer’s treatment.
Future research aims to harness new technologies such as advanced imaging techniques and genetic profiling to deepen insights into Alzheimer’s pathology. This approach seeks to elucidate the biological underpinnings of neurodegeneration, enabling the identification of effective therapeutic targets. Furthermore, by analyzing the interplay between microglial activity and synaptic health, researchers can develop more refined strategies that precisely address the mechanisms driving Alzheimer’s progression.
In addition, the increasing focus on early detection of Alzheimer’s is reshaping the landscape of treatment options. As scientists identify new biomarkers indicative of early disease mechanisms, the potential for timely interventions becomes more pronounced, allowing for earlier and more effective management of Alzheimer’s symptoms. The emphasis on proactive care reflects a broader shift in the medical community towards prevention rather than solely therapeutic responses.
Ultimately, the future of Alzheimer’s disease research holds great promise. Through sustained efforts in research, funding, and collaboration, there is hope for novel therapies that not only combat Alzheimer’s effectively but also enhance the quality of life for those living with neurodegenerative diseases.
Building Collaborative Networks for Alzheimer’s Research
The pursuit of solutions to the challenges posed by Alzheimer’s disease necessitates collaborative networks that bring together diverse expertise across various fields. These interdisciplinary efforts can catalyze discoveries and foster a rich exchange of ideas, crucial for advancing research on neurodegenerative diseases. Initiatives led by researchers such as Beth Stevens illustrate the power of collaboration, as teams work to integrate knowledge from neuroscience, immunology, genetics, and clinical practice.
Building robust partnerships enhances the ability to tackle the multifaceted nature of Alzheimer’s, pooling resources and knowledge to address gaps in research. For example, collaborations between academic institutions, healthcare providers, and industry can lead to the effective translation of laboratory findings into clinical applications. This interconnected approach promotes a comprehensive understanding of Alzheimer’s disease and expedites the journey from research to therapeutic innovation.
Moreover, these collaborative networks foster a culture of shared goals and collective problem-solving that can drive progress in Alzheimer’s research. By leveraging different strengths and perspectives, researchers can pursue more holistic solutions to complex issues. Such synergies not only lead to comprehensive research findings but also create a vibrant environment conducive to novel ideas and approaches.
In essence, building collaborative networks is vital for propelling Alzheimer’s research forward. The commitment to teamwork and interdisciplinary inquiry will lay the groundwork for significant advancements in understanding and treating neurodegenerative diseases, ultimately benefiting millions affected by Alzheimer’s.
Public Awareness and Education on Alzheimer’s Disease
Public awareness and education about Alzheimer’s disease are crucial components in the broader fight against neurodegenerative diseases. By informing the community about the symptoms, risks, and available therapies associated with Alzheimer’s, we can cultivate a more informed society that actively participates in prevention strategies and supports research initiatives. Educational campaigns play an essential role in dispelling myths about Alzheimer’s, empowering individuals to seek knowledge and assistance as early as possible.
Engaging the public in conversations about Alzheimer’s extends the urgency of research efforts. It fosters a climate where funding, resources, and attention are directed towards scientific inquiry. Furthermore, education initiatives can inspire individuals to advocate for policies that enhance support for those living with Alzheimer’s and their caregivers, thereby amplifying the need for effective treatments and academic research.
Moreover, understanding the complexities of Alzheimer’s disease, including its connection to aging and microglial dysfunction, equips the public to identify early warning signs and seek prompt medical advice. This proactive approach to health care can facilitate early diagnosis and intervention, leading to better management of symptoms and improved quality of life.
Overall, enhancing public awareness and education about Alzheimer’s is fundamental in shaping a society that prioritizes research and supportive resources for affected families. By promoting a culture of understanding around Alzheimer’s disease, we empower communities to engage with ongoing research and advocate for vital funding for emerging therapies.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s disease research?
Microglial cells are crucial components of the brain’s immune system and play a significant role in Alzheimer’s disease research. They patrol the brain, clear out dead or damaged cells, and prune synapses, which can affect neuronal communication. Aberrant pruning by microglia has been implicated in neurodegenerative diseases, including Alzheimer’s, highlighting their importance in ongoing research efforts.
How is Beth Stevens contributing to the fight against Alzheimer’s disease?
Beth Stevens is a prominent neuroscientist whose research on microglial cells has transformed our understanding of the brain’s immune system in relation to Alzheimer’s disease. Her findings on the aberrant pruning of synapses inform the development of new medicines and biomarkers that could lead to earlier detection and improved treatments for Alzheimer’s and other neurodegenerative diseases.
What impact does the aberrant pruning of synapses have on Alzheimer’s disease?
Aberrant pruning of synapses by microglial cells can lead to disruptions in neuronal communication, playing a significant role in the progression of Alzheimer’s disease. By understanding how these immune cells function and sometimes malfunction, researchers aim to develop targeted therapies that can restore balance and perhaps alter the disease’s course.
What are the future implications of Stevens’ research on microglial cells for Alzheimer’s treatment?
The research conducted by Beth Stevens on microglial cells holds promise for the future of Alzheimer’s treatment. By identifying how microglia contribute to neurodegenerative diseases, her work could lead to the creation of new therapeutic strategies aimed at correcting the mechanisms underlying Alzheimer’s, potentially improving outcomes for millions of affected individuals.
How do microglial cells contribute to the development of neurodegenerative diseases like Alzheimer’s?
Microglial cells are essential for maintaining brain health, but when they engage in aberrant pruning or respond improperly to signals, they can contribute to neurodegenerative diseases, including Alzheimer’s. Research is focusing on how targeting these immune cells could prevent or slow the progression of Alzheimer’s disease by restoring their normal functioning.
What is the significance of basic science in Alzheimer’s disease research according to Beth Stevens?
According to Beth Stevens, basic science is fundamental to advancing Alzheimer’s disease research. Her work demonstrates that understanding the underlying mechanisms of microglial function through curiosity-driven science is essential for developing innovative treatments for neurodegenerative diseases, including Alzheimer’s.
What are the expected trends in Alzheimer’s disease prevalence and research funding?
As the U.S. population ages, the prevalence of Alzheimer’s disease is expected to double by 2050, escalating the economic burden of care. In response, there is an increasing focus on research funding, such as that provided by NIH, which is essential for exploring new avenues in Alzheimer’s disease research, including the role of microglial cells.
How can research on microglial cells lead to earlier detection of Alzheimer’s disease?
Research on microglial cells is paving the way for the identification of new biomarkers related to Alzheimer’s disease. By understanding how these immune cells interact within the brain, scientists hope to develop methods for earlier detection, which can lead to timely interventions and better management of the disease.
| Key Points | Details |
|---|---|
| Beth Stevens’ Research | Focuses on microglial cells, which are the brain’s immune system and play a role in clearing damaged cells and pruning synapses. |
| Impact on Alzheimer’s | Stevens’ research shows that abnormal pruning by microglia is linked to Alzheimer’s disease and other neurodegenerative disorders. |
| Potential for New Treatments | Research may lead to the development of new medicines and biomarkers for early detection of Alzheimer’s disease. |
| Future Alzheimer’s Cases | With the aging U.S. population, cases of Alzheimer’s are projected to double by 2050, increasing care costs significantly. |
| Funding Sources | Mostly supported by federal agencies like the National Institutes of Health, emphasizing the importance of basic and curiosity-driven research. |
| Broader Scientific Implications | Basic research in animals can lead to significant findings and treatments for humans, as exemplified by Stevens’ work. |
Summary
Alzheimer’s disease research is crucial for understanding and combating one of the most pressing health concerns of our time. The groundbreaking work of Beth Stevens illustrates the importance of studying microglial cells and their role in brain health. By revealing how abnormal pruning by these cells contributes to Alzheimer’s disease, her findings pave the way for innovative treatments and early detection opportunities. As the number of Alzheimer’s cases continues to rise, ongoing support for research in this area is more important than ever.