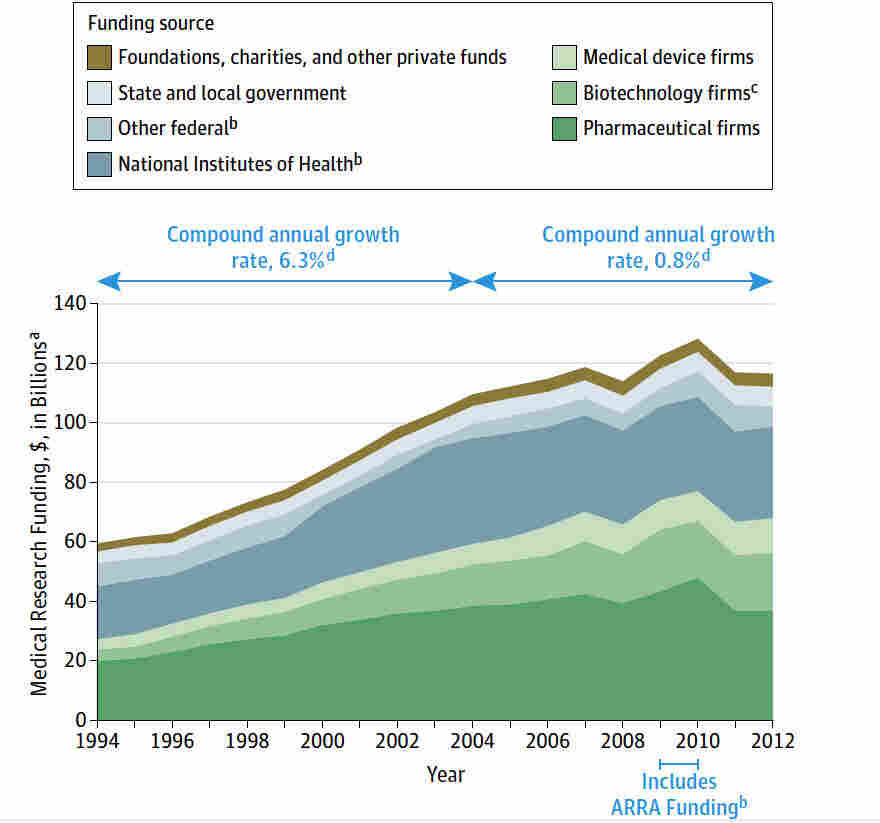
Medical research funding plays a critical role in advancing our understanding of diseases and improving patient safety in research. Unfortunately, recent cuts to federal research grants, particularly those from the National Institutes of Health (NIH), have raised serious concerns about the integrity of clinical trial oversight and the ethical conduct of studies. As funding sources dwindle, institutional review boards (IRBs) face increasing challenges in ensuring that research adheres to strict research ethics and provides adequate protections for participants. These oversight entities are essential in evaluating safety protocols and consent processes that safeguard patient welfare. The impact of NIH funding cuts extends beyond administrative hurdles, potentially jeopardizing the safety of individuals engaged in cutting-edge medical research.
The topic of medical research financing is becoming increasingly significant as we witness shifting governmental policies affecting the landscape of clinical trials. Specifically, federal funding streams play a crucial role in providing resources for the oversight of research activities, ensuring compliance with regulations aimed at protecting participant rights. Alternative funding mechanisms, such as private grants or institutional contributions, may not suffice in filling the gaps left by federal support. The importance of ethical standards, reinforced by institutional review boards, remains paramount in maintaining public confidence in medical studies. As we examine the future of clinical research, understanding the implications of funding fluctuations on patient safety and research oversight becomes essential.
The Effects of Funding Cuts on Patient Safety in Medical Research
The recent halt in funding for medical research has created significant challenges in maintaining patient safety during clinical trials. Without adequate financial resources, the infrastructure that supports research oversight—such as Institutional Review Boards (IRBs)—struggles to operate effectively. These boards play a crucial role in ensuring that every research study is ethically sound and that participant rights are prioritized. When funding is cut, the ability to monitor ongoing studies diminishes, leaving participants at risk. For example, when the SMART IRB received a stop-work order, it halted new site additions, thereby preventing numerous clinical trials from expanding crucial research that could lead to medical advancements.
Moreover, the interruption in funding disrupts the training and resources available to research professionals who are dedicated to safeguarding patient welfare. As the history of medical research shows, instances of unethical studies have resulted in distrust and skepticism among the public. This concerns regulators and the medical community because a reduction in oversight can lead to the kind of lapses in ethics and patient safety that modern regulations were designed to prevent. When participants feel uncertain about their safety, they may choose to withdraw from studies, ultimately stalling progress in critical research areas.
How NIH Funding Supports Ethical Oversight
NIH funding is essential for ensuring that medical research adheres to stringent ethical standards. With significant resources at their disposal, institutions are able to maintain robust IRB processes, ensuring patient rights are protected throughout research trials. This funding allows for systematic oversight of clinical trials, making certain that research proposals undergo rigorous evaluations for compliance with ethical standards. The NIH’s 2018 mandate for single IRB reviews delineated a clear pathway for the oversight of multisite research, enhancing efficiency without sacrificing participant safety.
Increased federal funding directly correlates with improved patient safety measures within research protocols. For instance, well-funded research teams can employ more thorough monitoring of clinical trial activities, which is essential for identifying adverse events quickly and effectively. This proactive approach to patient safety not only protects study participants during the trial but also sets a benchmark for ethical conduct that shapes future research practices across the country.
The Role of Institutional Review Boards in Clinical Trials
Institutional Review Boards (IRBs) serve as the backbone of ethical medical research, meticulously reviewing protocols to ensure the protection and well-being of participants. Their role extends beyond mere oversight; they are instrumental in assessing the risk-benefit ratios of studies and ensuring informed consent processes are properly executed. By having a dedicated focus on patient safety, IRBs help build public trust in medical research initiatives. Their expertise in navigating complex regulatory environments means that they can adapt to new challenges and uphold the highest standards for ethical research.
In many cases, IRBs also function as a liaison between research teams and participants, fielding questions and addressing concerns that arise during the trials. This communication bridge fosters a sense of transparency and ethical accountability in clinical studies. As history demonstrates, when IRBs are robustly funded and supported, they can avert potential ethical breaches, ensuring that the painful lessons of the past do not recur.
Clinical Trial Oversight: A Historical Perspective
The framework for clinical trial oversight has evolved significantly, shaped by historical precedents that have highlighted the consequences of unregulated research. From the unethical experiments conducted during World War II to the infamous Tuskegee Study, each historical episode has underscored the necessity of ethical oversight in protecting human subjects. These events catalyzed changes in policy and created the imperative for stringent IRB evaluations that we see today. By learning from past atrocities, the medical community strives to reassure participants and uphold their rights in contemporary research.
Understanding the historical context of clinical trials emphasizes the critical need for maintaining robust oversight mechanisms, such as IRBs. The trust that participants place in clinical researchers is easily eroded by past transgressions. Continuous education and training in research ethics help ensure that today’s researchers are equipped to implement ethical standards that foster participant safety and trustworthiness in medical research.
The Importance of Research Ethics in Patient Participation
Research ethics are fundamental in establishing guidelines for the treatment of participants in clinical trials. By adhering to ethical principles, researchers can ensure that individuals participating in studies are treated with respect, dignity, and care. Ethical frameworks dictate that participants should be fully informed about the nature of their involvement and the potential risks involved, fostering a culture of transparency and respect. When funding cuts threaten the integrity of these processes, the trust between researchers and participants may falter, affecting enrollment and ultimately the success of vital studies.
Additionally, ethical oversight through IRBs ensures that participant safety is a priority in all research activities. By systematically evaluating proposals for their ethical implications, IRBs safeguard against potential harms and encourage researchers to design studies that truly prioritize participant welfare. Upholding these ethical standards not only protects individuals but also enhances the overall credibility of clinical research as a whole.
Impact of Federal Funding on Research Collaboration
Federal funding serves as a catalyst for collaboration among research institutions, facilitating the sharing of knowledge and resources. Programs like SMART IRB exemplify how funding can streamline processes across institutions, reduce barriers, and promote innovative research. When funding is stable, research sites can confidently expand their collaboration, moving towards multisite studies that hold the promise of groundbreaking advancements in medicine and treatment. This cooperation is essential for tackling complex health challenges that no single institution could address alone.
However, when federal research funding is slashed, the ensuing disruption can lead to a contraction in collaborative efforts. Institutions may find themselves unable to support vital partnerships, resulting in member sites withdrawing from studies and terminating ongoing projects. This not only hampers scientific progress but also negatively impacts the communities that depend on advancements in healthcare. By understanding the critical relationship between federally funded research and collaboration, stakeholders can advocate for policies that bolster these essential financial resources.
Maintaining Patient Trust through Compliance and Oversight
One of the cornerstones of successful clinical research is maintaining the trust of participants, which can be significantly influenced by the integrity of compliance and oversight practices. Ethical oversight ensures that researchers adhere to stringent guidelines, thereby safeguarding participant safety and rights throughout the research process. By prioritizing compliance through a robust IRB framework, institutions can foster an environment where participants feel safe and valued, encouraging them to engage in future studies.
Trust is not easily restored once it has been broken, especially in light of historical misuse of research. Therefore, robust compliance measures, supported by adequate NIH funding, are essential for both fostering public confidence and ensuring participant retention in studies. In strengthening these practices, researchers can demonstrate their commitment to ethical standards and the well-being of individuals involved in clinical trials.
Expanding Access to Clinical Trials through Funding and Oversight
Access to clinical trials is critical for patients seeking new treatment options; funding can significantly influence availability and participation. Adequate funding enables research institutions to broaden their outreach, ensuring that diverse populations are represented in clinical studies. Additionally, funding provides for the necessary training and resources for IRBs, allowing them to effectively oversee clinical trials and assess participant recruitment strategies. Without sufficient financial support, efforts to engage disadvantaged communities may falter, perpetuating health disparities.
Furthermore, improving access to clinical trials not only benefits researchers but also enhances the likelihood of successful trial outcomes, ultimately leading to the development of safe and effective treatments. With a well-funded IRB structure, research institutions can proactively address potential barriers to access and ensure that all eligible participants have the opportunity to contribute to meaningful advancements in healthcare.
Addressing Concerns of Mistrust in Medical Research
Mistrust in the medical research community can pose significant challenges to patient recruitment and retention in clinical trials. Historical injustices, combined with stories of unethical research practices, have left many individuals skeptical about modern trials. To counteract this mistrust, it is imperative that IRBs and research teams actively engage with communities to address concerns and provide transparency regarding the research process. Adequate funding is crucial to afford outreach programs and educational initiatives that can help rebuild trust and assure participants of their safety and rights.
By integrating ethical review with open communication strategies, research institutions can foster an environment where participants feel informed and empowered to make decisions about their involvement. Engaging communities through meaningful discussions about research ethics and patient safety can bridge knowledge gaps, mitigating fears that stem from historical precedence. This proactive approach can enhance participation rates and ultimately improve the quality of medical research outcomes.
Frequently Asked Questions
How does medical research funding ensure patient safety during clinical trials?
Medical research funding plays a crucial role in ensuring patient safety during clinical trials by providing financial resources for the establishment and maintenance of Institutional Review Boards (IRBs). These boards review research proposals to guarantee that risks to participants are minimized and that ethical standards are upheld. NIH funding helps support these ethical oversight functions, which are essential to protect the rights and welfare of individuals participating in clinical studies.
What is the impact of NIH funding on research ethics in medical studies?
NIH funding significantly impacts research ethics in medical studies by mandating strict oversight through Institutional Review Boards (IRBs). These boards ensure compliance with ethical standards and regulations while safeguarding the welfare of research participants. The funding also supports training programs that educate researchers about ethical practices and patient safety, thus fostering a culture of integrity and accountability within the medical research community.
How do institutional review boards (IRBs) help in maintaining patient safety in research?
Institutional Review Boards (IRBs) are pivotal in maintaining patient safety in medical research by meticulously reviewing study designs, ensuring informed consent processes are transparent, and assessing potential risks to participants. IRBs, especially those backed by medical research funding, play a critical role in overseeing trials to guarantee ethical standards are met, thus protecting participants throughout the research process.
What are the consequences of funding cuts on clinical trial oversight?
Funding cuts can severely hinder clinical trial oversight by limiting resources available for Institutional Review Boards (IRBs) and regulatory compliance activities. This disruption can lead to lapses in monitoring, potential risks to participants, and a reduction in the overall quality and integrity of research processes, ultimately compromising patient safety and public trust in medical research.
How do funding disruptions affect the collaboration of institutions in medical research?
Funding disruptions, such as the halt in medical research funding, can significantly impair the collaboration between various research institutions. Without adequate resources, initiatives like SMART IRB, which facilitates multi-site research oversight, may be halted. This can lead to delays in research studies, inhibit innovation, and increase the risks to patients due to bottle-necked processes in clinical trial management and oversight.
What historical events led to the establishment of IRBs in response to medical research ethics?
Several historical events, including the Tuskegee Syphilis Study and unethical medical experiments during World War II, highlighted the need for ethical oversight in medical research. In response, IRBs were established to enforce standards of patient safety, informed consent, and research ethics. The establishment of these boards was further reinforced by the introduction of federal funding regulations, ensuring that all NIH-funded research adheres to strict ethical guidelines.
Why is it important to fund IRBs supporting patient safety in medical research?
Funding Institutional Review Boards (IRBs) is vital for supporting patient safety in medical research as it facilitates thorough ethical reviews and oversight of clinical trials. Properly funded IRBs are essential in ensuring that researchers implement necessary safeguards, obtain informed consent, and manage risks effectively, thereby promoting ethical standards and enhancing public trust in medical scientific endeavors.
In what ways does NIH funding impact the ethical landscape of medical research?
NIH funding impacts the ethical landscape of medical research by mandating comprehensive oversight and ethical reviews through IRBs, thus setting a standard for research integrity. The funding enables institutions to uphold high ethical standards, offer education on research ethics, and provide necessary resources for protecting patient safety, which are crucial for conducting credible and responsible medical research.
| Key Point | Details |
|---|---|
| Federal Funding Cuts | The Trump administration’s freeze of over $2 billion in research grants to Harvard has disrupted medical research efforts. |
| SMART IRB System | The SMART IRB system facilitates oversight of multi-site medical research but is now disrupted. |
| Role of IRBs | Institutional Review Boards (IRBs) ensure participant safety and rights in research studies. |
| Impact on Patients | Funding cuts could lead to halted studies, risking patient safety and public trust in research. |
| Historical Context | Past unethical research highlighted the need for strict oversight and led to the establishment of IRBs. |
| Ongoing Support | Harvard Medical School is currently supporting the IRB team to resume essential collaborative research. |
Summary
Medical research funding is crucial for the safety and protection of patients involved in clinical studies. The halt in funding, particularly the freeze applied to federal research grants, has had severe repercussions, disrupting oversight systems like the SMART IRB that are vital for patient safety. Continued efforts to secure adequate funding are necessary to maintain the integrity of medical research and foster public confidence in the safety protocols that protect participants. Without this support, research efforts may suffer, ultimately jeopardizing advancements in medical treatments and patient care.