TIM-3 therapy for Alzheimer’s is garnering attention as a promising approach to combating Alzheimer’s disease by harnessing immune system mechanisms traditionally exploited in cancer treatment. Recent studies reveal that blocking the TIM-3 checkpoint molecule can unleash microglia, the brain’s resident immune cells, allowing them to effectively clear amyloid plaques, which are hallmarks of Alzheimer’s. This innovative therapy could revolutionize Alzheimer’s disease treatment, offering new hope to millions affected by this debilitating condition. By enhancing microglial function, TIM-3 therapy not only targets the symptoms but also addresses the underlying causes of neurodegeneration, potentially improving cognitive abilities. With ongoing research, including genetic studies linking TIM-3 to late-onset Alzheimer’s, the future of this therapeutic strategy looks increasingly optimistic.
The exploration of TIM-3 therapy for Alzheimer’s disease marks a significant shift in how researchers approach neurodegenerative conditions. Leveraging an immune checkpoint strategy, similar to those used in cancer immunotherapy, scientists are investigating how the manipulation of specific molecules, such as TIM-3, can influence brain health. By understanding the role of microglia—the brain’s immune cells—researchers are uncovering potential pathways to restore cognitive function and mitigate the impacts of plaque accumulation. The intersection of immune system research and Alzheimer’s pathology opens new doors for innovative treatments that could redefine care for individuals suffering from this challenging disease. As checkpoint molecule research progresses, the hope for effective Alzheimer’s treatments becomes increasingly tangible.
The Promise of TIM-3 Therapy for Alzheimer’s Disease
Recent groundbreaking research has suggested that TIM-3 therapy may play a crucial role in the future of Alzheimer’s disease treatment. Leveraging an immune checkpoint molecule already used in cancer therapies, scientists are investigating its potential to enhance microglial function in the brain. Microglia are pivotal in the clearance of amyloid plaques, which are characteristic of Alzheimer’s pathology. By blocking the TIM-3 molecule, researchers have shown that microglia can regain their ability to attack and remove these damaging plaques, thereby improving cognitive function in experimental models.
The initial studies on TIM-3 therapy indicate not only a decrease in plaque formation but also a noteworthy restoration of memory functions in mice. This raises hopes that similar strategies could translate into effective treatments for humans facing Alzheimer’s disease. Given the genetic link between TIM-3 and late-onset Alzheimer’s, targeted therapies could provide a new direction in combating this neurodegenerative disorder. With continued research, we may soon see clinical trials exploring the efficacy of TIM-3 inhibitors, paving the way for innovative Alzheimer’s treatments.
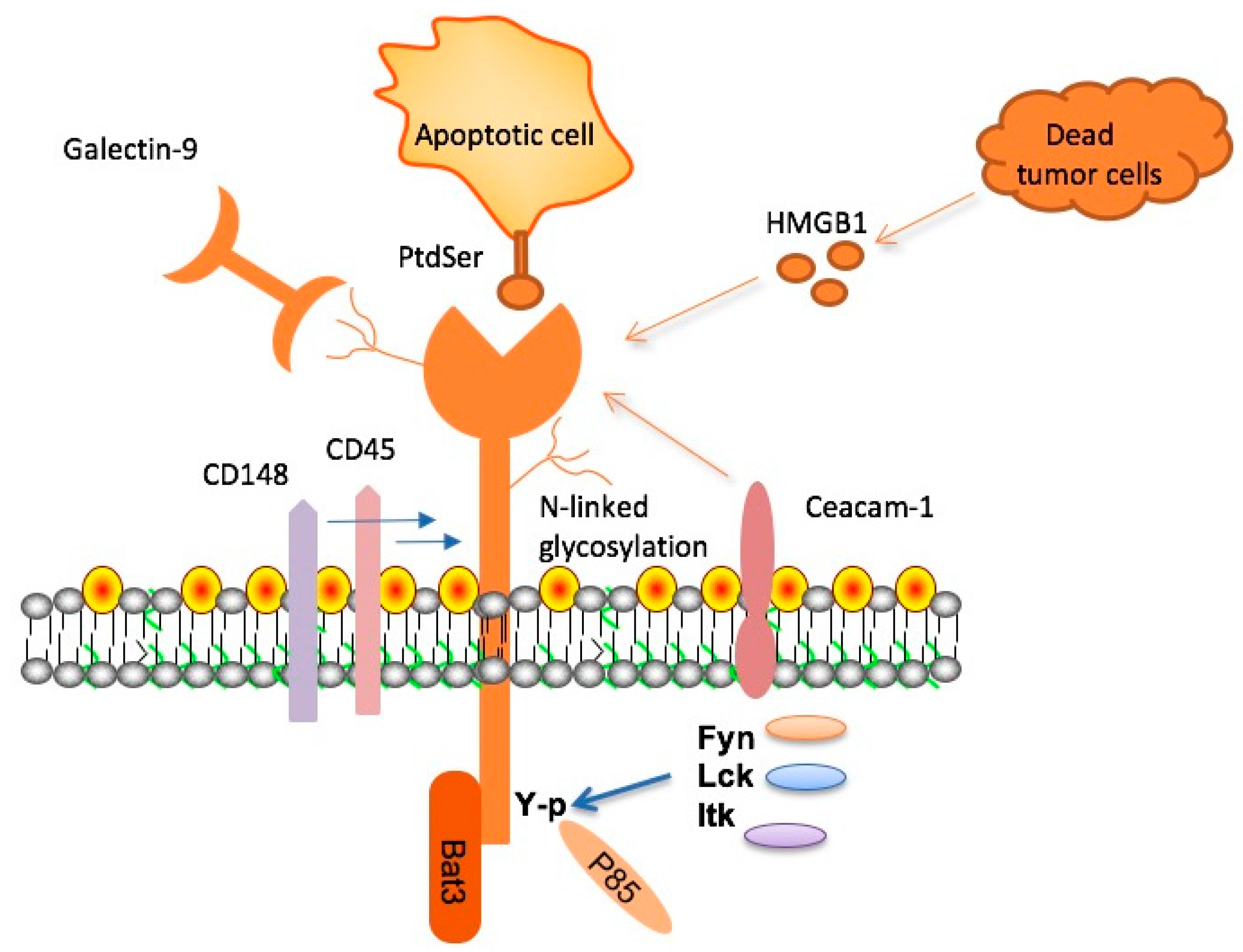
Understanding Microglial Function and Alzheimer’s
Microglia serve as the primary immune defense in the central nervous system, playing vital roles in both maintaining brain health and responding to damage. In Alzheimer’s disease, their functions become compromised, primarily due to the overexpression of TIM-3, which inhibits their ability to engage in plaque clearance. Research reveals that these cells, while essential in pruning synapses during development, become dysfunctional in aging brains, leading to an accumulation of amyloid-beta plaques. Consequently, the impaired function of microglia in this context may contribute significantly to the progression of Alzheimer’s disease.
By understanding the mechanisms that underlie microglial function, researchers are uncovering potential therapeutic targets, such as TIM-3. This molecule has been shown to dysfunctionally regulate microglial activity, inhibiting their ability to phagocytose harmful plaques. Recent studies emphasize the dual role of microglia, where they not only protect the brain but, if activated incorrectly, can contribute to neurodegenerative processes. These insights not only enhance our understanding of Alzheimer’s pathogenesis but also illustrate the potential for immune-targeted therapies to modulate these cells for therapeutic benefits.
Checkpoints Molecules and Their Role in Neurodegeneration
Checkpoint molecules, such as TIM-3, originally identified for their role in cancer treatment, have gained interest in neurodegenerative research. Their primary function is to maintain immune balance—preventing excessive immune responses that can lead to tissue damage. In the context of Alzheimer’s disease, it appears that these molecules may inadvertently hinder the brain’s immune cells, particularly microglia, from clearing toxic amyloid plaques. The role of TIM-3 as a negative regulator thus represents a unique intersection of cancer immunology and neurodegenerative disease treatment.
The potential to manipulate checkpoint molecules for therapeutic gain extends to novel Alzheimer’s strategies aimed at reinvigorating microglial activity. As researchers shift focus toward the immunological aspects of Alzheimer’s intervention, TIM-3 inhibitors may offer a pathway to improving cognitive function. This evolving understanding of checkpoint molecules not only informs future clinical approaches but also expands the landscape of possible interventions for Alzheimer’s, thereby revitalizing hope in the quest for effective treatments.
Exploring Genetic Links: TIM-3 and Alzheimer’s Disease
Genetic studies have underscored the significance of TIM-3 in the context of late-onset Alzheimer’s disease, highlighting its role as a risk factor. The polymorphism associated with the TIM-3 gene (HAVCR2) provides critical insight into why some individuals are more susceptible to developing Alzheimer’s. Elevated expression levels of TIM-3 in microglia of Alzheimer’s patients suggest that genetic predisposition can lead to the dysfunctional behavior of these immune cells, culminating in the failure to clear amyloid plaques.
Understanding the genetic underpinnings associated with TIM-3 opens new avenues for personalized medicine in Alzheimer’s treatment. By identifying specific genetic markers linked to TIM-3, researchers can potentially develop tailored therapies that target these pathways in at-risk populations. Such advancements could not only aid in the prevention of disease progression but also improve outcomes for patients already affected by Alzheimer’s, emphasizing the importance of genetic factors in shaping therapeutic strategies.
Current Advances in Alzheimer’s Research
The intersection of cancer immune therapy techniques and Alzheimer’s disease represents a promising frontier in medical research. Recent studies have begun to unravel the complexity of using strategies developed for cancer, such as TIM-3 inhibition, in combating neurodegenerative diseases. By turning off inhibitory checkpoint molecules, researchers aim to revitalize the capacity of microglia to clear amyloid-beta plaques, a hallmark of Alzheimer’s. This innovative approach not only showcases the versatility of immune therapies but also highlights a potential paradigm shift in how we conceive of and treat Alzheimer’s pathology.
Moreover, the remarkable adaptation of existing therapies, tested initially in oncology, demonstrates the evolving nature of biomedical research. As we witness a growing body of evidence supporting TIM-3’s role in Alzheimer’s, understanding its regulation could offer crucial insights into designing novel therapeutic strategies. With clinical trials on the horizon, the commitment to exploring these avenues raises hope for effective Alzheimer’s treatments that could significantly enhance patient quality of life.
Challenges in Alzheimer’s Drug Development
Despite promising discoveries, developing effective Alzheimer’s treatments remains fraught with challenges, particularly in navigating the biological intricacies of the disease. The failure of many high-profile drug trials has led to skepticism regarding novel therapies, yet recent successes provide a glimmer of hope. The potential of drug candidates targeting TIM-3 represents an optimistic trajectory within a landscape characterized by trial and error, signaling the need for continued investment in research and development.
Equally important is the consideration of practical implications for translating laboratory findings into clinically viable treatments. Researchers must address how TIM-3-targeted therapies can be effectively delivered to the brain, overcoming barriers such as the blood-brain barrier. Clinical studies focused on this checkpoint molecule may yield insights that not only deepen our understanding of Alzheimer’s but also reshape therapeutic targets, paving the way for more significant advancements in treating this pervasive disease.
The Future of Alzheimer’s Disease Therapy
Emerging therapies centered around TIM-3 inhibition signify a new era in Alzheimer’s research, with ongoing studies poised to evaluate their efficacy in humans. The exploration of small molecules and antibodies that aim to manipulate the immune response offers promising avenues for improving patient outcomes. By targeting TIM-3 and enhancing microglial function, these therapies could potentially halt or reverse cognitive decline associated with Alzheimer’s.
As we look to the future, the integration of genetic research with advances in immunotherapy will be essential in shaping personalized approaches to Alzheimer’s treatment. The ongoing collaboration between researchers and the clinical community will facilitate the transition from bench to bedside, ensuring that promising concepts such as TIM-3 therapy are tested and refined for human applications. By maintaining a steadfast focus on innovation and adaptability, the fight against Alzheimer’s disease may finally witness transformative breakthroughs.
The Role of Collaboration in Alzheimer’s Research
The complexity of Alzheimer’s disease necessitates collaborative efforts among scientists, clinicians, and researchers across various disciplines. The research surrounding TIM-3 therapy illustrates the power of interdisciplinary collaboration in unlocking therapeutic potential. By combining expertise in immunology, neurology, and genetics, teams can develop comprehensive approaches that address the multifaceted nature of Alzheimer’s.
Such collaborations not only enrich the quality of research but also accelerate the pace of discovery, as seen in the recent advancements concerning TIM-3 in Alzheimer’s. As multidisciplinary teams unite to tackle this global health crisis, they can share insights and resources, fostering innovation and enhancing the likelihood of successful treatment outcomes. The strength of collaboration will undoubtedly play a pivotal role in advancing Alzheimer’s research and developing effective therapies.
Public Awareness and Alzheimer’s Research
As research into TIM-3 therapy and its implications for Alzheimer’s disease progresses, public awareness about the disease and its treatments becomes increasingly vital. Raising awareness helps engage broader support for research funding and encourages participation in clinical trials, which are crucial for testing new therapies. Understanding the importance of ongoing research in Alzheimer’s can inspire communities to become involved and make a positive impact on patients and caregivers alike.
By creating a more informed public, we can foster a supportive environment for research initiatives. The role of community engagement in promoting Alzheimer’s awareness and the importance of TIM-3 therapy in breakthrough treatments can not be understated. Grassroots efforts, educational campaigns, and dialogue about Alzheimer’s objectives can ultimately contribute to a deeper understanding of the disease, spur donations toward research initiatives, and support those navigating the journey of diagnosis and treatment.
Frequently Asked Questions
What is TIM-3 therapy for Alzheimer’s and how does it work?
TIM-3 therapy for Alzheimer’s involves targeting the TIM-3 molecule, which is an immune checkpoint that inhibits microglia, the brain’s immune cells, from clearing amyloid plaques associated with Alzheimer’s disease. By blocking TIM-3, microglia can attack and degrade these plaques, potentially restoring cognitive function.
How is TIM-3 related to late-onset Alzheimer’s disease?
Research shows that TIM-3 is linked to late-onset Alzheimer’s disease as a genetic risk factor. A polymorphism in the TIM-3 gene, known as HAVCR2, is found more frequently in patients with late-onset Alzheimer’s.
What role do microglia play in Alzheimer’s disease and TIM-3 therapy?
Microglia are the brain’s immune cells responsible for clearing plaques. In Alzheimer’s disease, TIM-3 inhibits microglia from effectively performing this function. TIM-3 therapy aims to lift this inhibition, allowing microglia to clear amyloid plaques and reduce memory loss.
What are the experimental results of TIM-3 therapy in mice with Alzheimer’s?
In studies, mice genetically modified to lack TIM-3 showed significant improvements in cognitive function and plaque clearance. This indicates that targeting TIM-3 can restore some memory functions and change plaque behavior, hinting at a potential breakthrough in Alzheimer’s treatment.
Can TIM-3 therapy for Alzheimer’s use existing cancer treatments?
Yes, TIM-3 therapy can repurpose existing anti-TIM-3 antibodies, initially developed for cancer treatment, to target Alzheimer’s disease effectively. This approach leverages the selective expression of TIM-3 on microglia, making it a promising strategy for combating Alzheimer’s.
What are the potential effects of blocking TIM-3 in Alzheimer’s therapy?
Blocking TIM-3 in Alzheimer’s therapy may enhance the ability of microglia to clear amyloid plaques, which could lead to improved cognitive function and potentially slow disease progression.
What challenges exist with TIM-3 therapy for Alzheimer’s disease?
One challenge is ensuring that anti-TIM-3 treatments effectively reach the brain without causing vascular damage, as seen with some antibody therapies targeting amyloid plaques. Ongoing research aims to optimize delivery methods for TIM-3 therapy.
What does the future of TIM-3 therapy look like for Alzheimer’s patients?
The future of TIM-3 therapy for Alzheimer’s looks promising as researchers are currently testing human anti-TIM-3 in animal models with the goal of preventing plaque development in Alzheimer’s disease. Further studies are needed to evaluate its effectiveness and safety in humans.
How does TIM-3 affect the immune response in Alzheimer’s disease?
TIM-3 modulates the immune response by restraining microglia from excess activity. While this is protective under normal conditions, in Alzheimer’s disease it hampers the clearance of harmful plaques, suggesting that TIM-3 therapy could help re-balance this response to benefit patients.
How long has research into TIM-3 therapy for Alzheimer’s been ongoing?
Research into TIM-3 therapy for Alzheimer’s has been ongoing for five years, highlighting the commitment to understanding its potential in reversing cognitive decline associated with the disease.
| Key Points | Details |
|---|---|
| Study Overview | Research indicates TIM-3 therapy could leverage immune checkpoint strategies used in cancer treatment to combat Alzheimer’s. |
| TIM-3 Mechanism | TIM-3 is a checkpoint molecule that inhibits microglia from clearing amyloid plaques in the brain. |
| Late-Onset Alzheimer’s | 90-95% of Alzheimer’s cases are late-onset, linked to TIM-3 genetic polymorphism. |
| Microglia Function | Microglia are brain immune cells that prune synapses and clear debris, but TIM-3 inhibits their function in Alzheimer’s. |
| Research Results | TIM-3 deletion in mice improved plaque clearance, enhanced cognition, and restored memory. |
| Potential Therapy | Using anti-TIM-3 antibodies may lead to new treatments for Alzheimer’s by enhancing microglial activity. |
| Next Steps | Ongoing studies aim to test human anti-TIM-3 antibodies in mouse models to observe effects on plaque development. |
Summary
TIM-3 therapy for Alzheimer’s is a promising approach that seeks to exploit mechanisms used in cancer treatment to improve cognitive function in those affected by late-onset Alzheimer’s disease. By understanding how TIM-3 inhibits microglial activity—the immune cells responsible for clearing plaque in the brain—the research suggests that targeting this pathway can potentially reverse cognitive decline associated with Alzheimer’s. Continued investigations into anti-TIM-3 antibodies aim to provide new avenues for treatment, offering hope for better management of this debilitating condition.